In this guide to Hard Metal (also known as Widia, Cemented Carbide or Tungsten Carbide) we define it as a material used in mechanical processing that consists of hard particles of Tungsten Carbide embedded in a metal matrix (often cobalt).
Hard metal is produced thanks to the sintering process ; in other words, the fine powders of the components are mixed, pressed and then heated while maintaining a high pressure so that the granules of the powders unite to form a single piece. This means that hard metals are not actual metals, but carbides (80-95%) bound by a metal.
The tungsten carbide cemented is the preferred material for all those parts that must withstand arduous any action such as abrasion , erosion , corrosion and metal seizure . For this reason, a hard metal guide is essential to guide technicians and designers.
In addition to a high pressure force, resistance to deformation at high temperatures. These are physical characteristics that are particularly useful in metal cutting applications. It allows a long life to tools which, if made with other materials, would be worn prematurely.


In the late 1800s, French chemist and 1906 Nobel laureate in chemistry Henri Moissan made an interesting discovery .
The chemist discovered that by mixing the tungsten powders with the carbon ones, a new compound was formed. A compound which, when heated at high temperature in an oven to its design electric arc, formed a material very hard and resistant to wear.
However, it was too brittle a material to be used in the typical applications of today’s hard metals. This problem was analyzed and solved by Karl Schroter in 1914. Schroter was working at the time at the Osram firm in Germany as a researcher.
His research was very specific and concerned the possibility of finding new materials for drawing the filaments of electric bulbs. The wire inside the bulb was made of a steel die which, over time, widened as the matrix of the wire gauge was worn.
In other words, at the beginning of the processing period the drawing was smaller, becoming bigger and bigger during the processing. Schroter was commissioned to find a stronger material than steel in order to draw the tungsten wire.
In this research he found the solution by mixing tungsten and cobalt : an intuition that led him to a new material. He discovered that it was possible to mix tungsten carbide powders with a metal binder such as nickel or cobalt and, then, the mixture could be sintered at a temperature of about 1500 ° C. In this way a low porosity product was obtained, with a very high hardness and good toughness.
Here is how this chemist came to discover a new alloy starting from a practical problem related to the production of light bulbs. This material was introduced for the first time, as a cutting tool, by Krupp (German steel industry) in 1927, with the registered name ” Widia ” (wie Diamant – like diamond).
In Germany, Friedrich Krupp bought the original patent and embarked on a Widia production program which consisted mainly of tungsten carbide particles intersected with a cobalt matrix consisting of 5 to 15% of the total composition.
Following tight negotiations with Krupp, all rights later passed to the Americans of General Electric, while Krupp retained the right to export cemented carbide to the United States. General Electric formed the Carboloy Company, which opened the Firth-Sterling Steel Company and Ludlum Steel Company subsidiaries. At that time in America cemented carbide was known under the terms Carboloy, Dimondite, and Strass Metal.
Although the first production tests were done in the Essen laboratories in 1922, it was only in 1926 that Krupp began trading Widia in Germany.
In the 1920s and 1930s, cemented carbide was very expensive , costing more than 450 euros per ounce. However, even at that price its use could be economically justified.
Moreover, the custom of making only the tip of the cemented carbide instruments derives precisely from economic considerations. In any case, the Widia instruments were tested in the General Electric plants and imposed themselves on public opinion in 1928.
Hard metal has truly unique characteristics . In these pages we will deepen this topic from a purely technical point of view.
First of all, the “hardness” of Widia must certainly be indicated. This is the physical property considered most important for practical applications. Although, as we shall see, it is not the only reason that has determined its business success, its resistance to abrasion is extraordinary.
Hardness is calculated using the indentation of a specimen pierced with an ASTM standard B-294 penetration diamond. The hardness values of Widia are expressed in terms of Rockwell “A” or Vickers values. In nature, the only material harder than this type of metal is diamond: only diamond is able to scratch the carbonate carbide. Silver and gold, in comparison, are much softer metals.
Another distinguishing feature is its density. This property is calculated with the ASTM B311 standard . The density of cemented carbide varies according to its composition being a composite alloy and, therefore, its constituent gradients have single variable densities.
By combining these materials in different proportions it is possible to create a variation in the density of the resulting matter. A density of 14.5 g / cc is typical for a 10% cobalt blend. This value has twice the density of wrought iron 1040: an element to be kept in mind especially when weight is an important factor in a practical application.
The mechanical strength of cemented carbide is typically determined by the transverse breaking strength method rather than a tensile test as is commonly done for steel.
This methodology is used because brittle materials are very sensitive to tensile test misalignment and surface defects, which could cause a stress concentration and lead to incorrect test results. The transverse breaking force is determined by placing a standard sample (for ASTM B-406, ISO 3327 ) between two supports and loading it to the breaking point. The value obtained is called the transverse breaking force or cohesion force and is measured in relation to the weight that caused it to break.
This test detects the load on the single area of the unit and is expressed in psi or N / mm2. Given that the cemented carbide has a range of fracture values differentiated by the existence of micro-voids, characteristic of all friable materials, this test is carried out by carrying out several tests: the resulting reference value is evaluated on the average of all tests observed.
The values for the transverse breaking force that appear in the property charts provided by the manufacturers reflect the mechanical force operated only for a specific area. Erroneously, many engineers – even those working in the metal industry – take this value as a model strength value.
This data is then used to evaluate to what degree the alloy should work in a particular application, expecting a direct correspondence with this value. In reality, these results decrease as the size of the area in question decreases: the value of the strength of the model should be calculated in relation to its actual size.
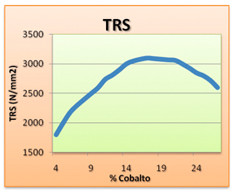
Another factor affecting the mechanical properties of cemented carbide, in particular the transverse breaking force, is its granular size . The more the size of the granulate increases, the more the transverse breaking force and the wear resistance decrease.
This is another of the most important attributes of cemented carbide. Ductile materials under compression simply swell or expand without fracture, but a brittle material does not withstand this type of test for the occurrence of shear fractures more than for true compression.
Cemented carbide exhibits a high level of compressive strength when compared to most other materials and the value increases with decreasing compound content and granulate size. Regarding the size of the granulate and the content of the compound, values between 400K-900K psi (7kN / mm2) are typical for cemented carbide.
Cemented carbide exhibits surprising impact strength , especially at high temperatures as it contains 25% binder cobalt with a coarse granular structure. Transverse failure is often misused as a measure of impact strength when, in fact, fracture strength is a better indicator of the cemented carbide’s ability to withstand any mechanical shock or impact. The fracture strength varies according to the size of the granulate and the binder contained.
When a material is subjected to repeated cycles of fluctuation, various damage can occur. These problems can occur, even if the material experiences less stress than would have been caused if the load stress had been constant.
The fatigue-related properties are evaluated by subjecting some sample samples to a stress cycle and the numbers of cycles that take place until damage are calculated. Several large companies have conducted this type of cemented carbide tests and have written their reports on it.
Swedish company Sandivik, for example, has verified that the fatigue strength of cemented carbide in a load compression can result in between 65% and 85% of the compressive force at 2 x 106 cycles. The fatigue strength increases as the size of the tungsten carbide granulate decreases and the binder content decreases.
The tungsten carbide particles are resistant to the most corrosive substances. It is a binding material which is subject to leaching in the presence of a strong acid or alkaline solution. The bonding material will leach off the hard metal surface, leaving a skeletal, unsupported structure.
The carbide particles will be scraped off rather quickly, exposing a new surface area that can be attacked. When the binder is low, the carbide skeleton is denser . A low binder grade shows a slightly higher combination of wear and corrosion resistance than those with a higher binder content.
These particles are also hard to crumble or weld and are used in specific applications where corrosion and wear resistance are an indispensable necessity while resistance to mechanical strength and thermal shock are so important.
Carbide shows a very low Linear Expansion Coefficient . About half that of steel. A degree of hard metal with l ‘8% cobalt indicatively has a linear expansion coefficient of 5 * 10-6 / ° C in a temperature range from 20 to 400 ° C . Thermal conductivity is approximately twice that of unalloyed steel and one third of that of copper. The specific heat capacity of a generic grade of hard metal is about 150-350 J / (Kg * ° C) , that is, about half of that of an unalloyed steel.
Carbide has a low electrical resistivity and a typical is 20 µOcm. As a consequence of the low resistivity, hard metal is a good conductor, having a conductivity value that is about 10% less than copper. Due to the cobalt or nickel content, the hard metal also exhibits ferromagnetic properties at room temperature.
Therefore the Curie temperature is included in the range between 950 and 1050 ° C , it depends on the composition of the grade.
it is very low and is a function of the cobalt content. It increases with the cobalt content. A typical value is in the range of 2 to 12 when the vacuum value is equal to 1.